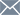24% Flavones 6%Ginkgolides Ginkgo Biloba Extract
Latin Name: Ginkgo biloba L.
Active Ingredients:Flavone 24%, Lactones 6%
Used Part:Leaf
Appearance:Brown Fine Powder
Specification
Total Ginkgo flavone Glycosides> 24% by HPLC
Total Terpene Lactone> 6% by HPLC
Acid≤5ppm or Acid≤1ppm
CAS No.: 90045-36-6
Specification of Ginkgo Biloba Extract
|
Ginkgo Biloba Extract 24/6 |
Flavones ≥ 24% , Lactones ≥ 6% |
|
Ginkgo Biloba Extract EP6 |
Flavones : 22% - 27% , Lactones : 5.4% - 7.0% BB : 2.6% - 3.2% , Ginkgolic Acid ≤ 5ppm |
|
Ginkgo Biloba Extract USP31 |
Flavones : 22% - 27% , Lactones : 5.4% - 12.0% Lactones A+B+C : 2.8% - 6.2% , BB : 2.6% - 5.8% Quercetin/kaempferol : 0.8-1.5 , Ginkgolic Acid ≤ 5ppm |
|
Ginkgo Biloba Extract CP2005 |
Flavones ≥ 24% , Lactones ≥ 6% Quercetin/kaempferol : 0.8-1.5 Ginkgolic Acid ≤ 5ppm , ≤ 10ppm |
|
Ginkgo Biloba Extract CP2010 |
Flavones ≥ 24% , Lactones ≥ 6% Quercetin/kaempferol : 0.8-1.2 Isohamnetin/kaempferol ≥ 0.15 Ginkgolic Acid ≤ 5ppm, ≤ 10ppm |
|
Ginkgo Biloba Extract Water Soluble |
Flavones ≥ 24% , Lactones ≥ 6% Ginkgolic Acid ≤ 5ppm , Solubility : 20:1 |
Function of Ginkgo Biloba Extract
- Ginkgo Biloba Extract may Improve Cognition.
- Ginkgo Biloba Extract helps Fight Free Radicals.
- Ginkgo Biloba Extract helps Boost Blood Circulation.
- Ginkgo Biloba Extract may Provide Other Health Benefits.
1. Applied in health product field, ginkgo biloba extract can effectively reduce breast pain and emotional instability.
2. Functional foods areas, ginkgo biloba extract has effect on protecting vascular endothelial tissue, regulating blood lipids.
3. Applied in pharmaceutical field, ginkgo biloba extract can be used for treating stomach-ache, diarrhea, high blood pressure, nervous and respiratory diseases such as asthma, bronchitis.